By Dr Corinne Smith, reader in structural biology and biophysics, and director of the Research Technology Platform in Advanced Bioimaging at the University of Warwick. Dr Smith was recently awarded a Royal Society Leverhulme Trust Senior Research Fellowship for her work on clathrin.
I am intrigued by a protein called clathrin. It consumes my interest in a unique way and has done for quite a large number of years. Why would something as functional as a protein prove such an attractive object of study?
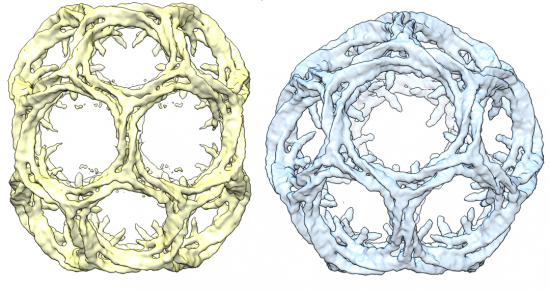
Clathrin cage structures. Kyle Morris and Corinne Smith, University of Warwick
The first reason is that it is actually attractive to look at. It has the unusual property of being able to form geometric lattices both as part of its function in cells and in a test tube as purified protein. Geometric structures are surprising to see in nature. When they do occur they look, well, unnatural. Just look at the lattice structures formed by these fungi, indusiata and clathrus ruber.

Indusiata and clathrus ruber
We expect biological material to be messy and irregular, and of course this often appears to be the case.
But look more closely and you discover that there is a system generating biological matter that follows physical and chemical laws as strictly as any builder or engineer. The processes are just way more complex. So, when we look at the biology, say of the enzyme lysozyme from some gloopy egg white, we see that the amino acid chain it is composed of forms intricate, highly organized patterns of helices and zig-zag strands.
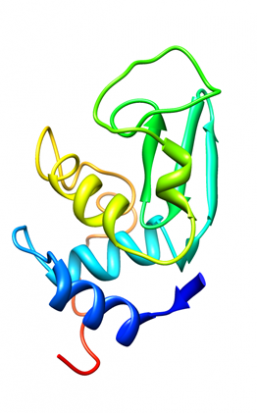
3D path of the protein chain of hen egg white lysozyme which forms helical structures. The arrow motif represents zig-zag structures called beta-strands. Diamond, R. (1974) J.Mol.Biol. 82: 371-391 1LYZ.pdb
Clathrin doesn’t only interest me for the way it looks, it is involved in an intricate mechanism of cellular transport called endocytosis. Clathrin helps cells to absorb nutrients like cholesterol and other required molecules and take them to where they are needed. To do this, the required molecule sticks to a protein embedded in the cell membrane called a receptor. Clathrin helps the cell to engulf this portion of the membrane into a lipid vesicle, which is detached and transported to its next destination. This is process is called clathrin-mediated endocytosis, which is one of several different systems of endocytosis used by cells.
In fact organisms make use of endocytosis to do much more than simply import nutrients. This type of biological machinery also supports the function of neurons, helps cells send chemical messages to control their function and enables newly made cells develop the features they need for more specialised work. So understanding in detail how endocytosis works contributes to our knowledge of many different aspects of the life of a cell.
Described like this endocytosis seems like rather a simple mechanism but clathrin is just one of many proteins that work together to make this happen. As well as forming lattice structures around these lipid vesicles, clathrin needs to interact efficiently with a complex protein network to perform its role. How it manages to do this is the current focus of my research. We take pictures of clathrin molecules in ice using an electron microscope and analyse them. These pictures reveal very detailed information about the precise internal structure of clathrin and we hope to be able to see in 3D detail how clathrin interacts with the network of proteins it coordinates. Getting a detailed picture like this will help us see how this system works and might even lead to new ways to control endocytosis. This could in turn help development of new therapies for diseases, including some cancers, familial hypercolesterolaemia and Alzhemier’s disease, where endocytic mechanisms play a role.

